Hybrid technologies: examples of applications Medical and health applications Membrane technology is of major importance in medical applications, particularly in a number of life-saving treatments. Membranes are amongst others used in drug delivery, artificial organs, tissue regeneration, diagnostic devices, bioseparations, and as coatings for medical devices. The total membrane area produced for medical applications almost equals the membrane area of all industrial membrane applications together. In all cases, biocompatible, and in some applications biodegradable materials are required for membrane fabrication. Drug delivery The goal of an ideal drug delivery system is to deliver a drug to a specific site at a specific moment and with a predefined release pattern. A constant drug level in blood or sustained drug release to avoid multiple doses and bypassing of the hepatic ‘first-pass’ metabolism forms an important challenge. In most membrane-based drug delivery systems, a drug reservoir is contained in a membrane device. In transdermal drug delivery, the drug is incorporated in a patch and delivered through the skin due to a concentration gradient or another driving force such as an electrical current. This allows continuous administration of drugs with short half-lives, rather similar to intravenous infusion, with the clear advantage that transdermal drug delivery is non-invasive and does not require hospitalization. |
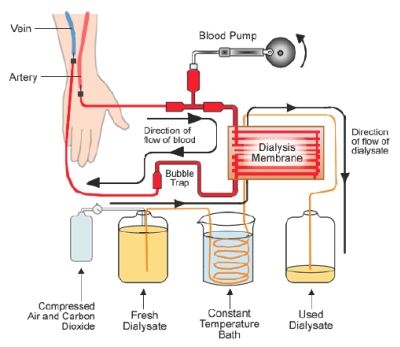
Dialysis - artificial kidney Healthy kidneys form an essential part of the metabolic processes of the human body. Kidney failure, due to infections, high blood pressure, diabetes or extensive use of medication, results in building up of harmful wastes and excess fluids in the body. Chronic kidney failure therefore requires a dialysis treatment using an artificial kidney. Blood is taken out of the body and passes through a special membrane that removes waste and excess fluids. The purified blood is then returned to the body. The dialysis machine can also administer drugs, for instance heparin to avoid blood clotting during the treatment. Today, more than 1.8 million people worldwide require regular kidney therapy, and among them 1.5 million undergo dialysis. A typical treatment involves 3 sessions a week which take 3-5 hours each. The yearly growth of dialysis patients amounts to 7-8%. |
 |
 |
A dialysis membrane contains pores that allow small molecules such as water, urea, creatinine and glucose to readily pass through, while retaining the red cells, platelets and most plasma proteins. Three treatment modes are commonly used, i.e. hemodialysis, hemofiltration and hemodiafiltration, in which solute removal is basically performed by diffusion, convection, and diffusion/convection, respectively. Most of the dialysers up to the late 1960s were manufactured from regenerated cellulose, often chemically modified to enhance blood compatibility. Later on, synthetic membranes were prepared from hydrophilic or hydrophilized copolymers (e.g. polymethylmethacrylate) or hydrophilic blends (e.g. polysulfone or polyethersulfone mixed with polyvinylpyrrolidone). The first dialysis membranes were flat plate-and-frame modules. Today, hollow fiber modules are mostly used. The technology of dialysis module preparation has advanced much and is nowadays fully automated. The membranebased therapy is still not complete and manufacturers, often in collaboration with academia, work on the improvement of membranes, modules and devices. |
Membranes in tissue engineering The replacement of organs has since long been subject of debate, however, the field of engineering tissue in vitro to repair damaged tissue in vivo arose only two decades ago. An autogenic tissue engineering transplant, using the patient’s own cells, would address most limitations of direct transplantation. Therefore, in vitro construction of engineered tissue replacements can offer an excellent alternative. One of the major research themes is scaffold fabrication. |
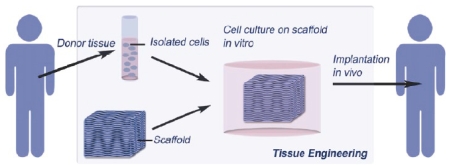 |
A scaffold is a 3-D construct that serves as temporary support for isolated cells to grow into a new tissue prior to implantation in the host tissue of the patient. The scaffold design determines to a large extent the functionality of the construct. The scaffold should be highly porous with good pore interconnectivity to ensure sufficient nutrient transport towards the cells and removal of waste products. In ‘soft’ tissue applications, e.g. skeletal muscle or cardiovascular substitutes, mainly polymers are used (e.g. collagen, fibrin, polyhydroxybutyrate, chitosan, polylactic acid) whereas ceramics and metals are especially applied in ‘hard’ tissue replacements, e.g. bone substitutes. A great variety of well-known membrane fabrication techniques are used in tissue engineering, in particular for scaffold fabrication. Since the field of tissue engineering is quite young, the broad clinical application of tissue engineering constructs is still premature. Nevertheless, a wide variety of materials have been tested as skin grafts and series of clinical products are available on the market. |