Pressure driven processes, technologies and membranes
|
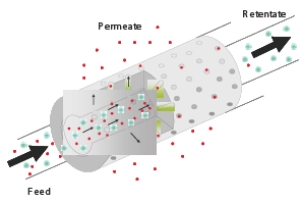
• Flux: The flux of a component (J) is the amount passing through the membrane per unit of time and per unit of membrane surface area. The flux divided by transmembrane pressure (TMP) yields the permeability (l h-1 m-2 bar-1) or MTC (mass transfer coefficient) value. The flux and has a strong impact on the capital costs of the process (~ membrane surface area needed) and hence influences the economic feasibility. • Selectivity or retention: Selectivity is the partition of a component into permeate and feed. For example, a retention of 80% for a component means that the concentration in the permeate is a factor of 5 lower compared to the feed concentration. In fact, selectivity will strongly determine the technical feasibility of a membrane separation process. The membrane itself can have different shapes (flat or tubular) and different structures (symmetric or asymmetric). In applications, they are mounted in modules and linked together, thus connecting incoming and outgoing streams. Most flat membranes are implemented in a spiral wound module, a design with a low cost and high surface to volume ratio. The specific configuration also determines the ease of cleaning. Configurations with tubular membranes are the easiest to clean and spiral wound the most difficult. Consequently, tubular membranes are applied in process streams with a high load or a high viscosity. Membrane operation During the course of a membrane separation process, membrane performance can decrease considerably over time. Such behaviour is mainly due to concentration polarisation and fouling. Concentration polarisation is a phenomenon related to the concentration increase near the membrane surface. Membrane fouling relates to a wide array of complex phenomena linked to the deposition of components at the surface and inside the membrane. Major fouling phenomena are particulate and colloidal fouling, scaling (precipitation of inorganic salts), bio-fouling and adsorption. |
Several approaches to reduce fouling can be distinguished, such as pretreatment of the feed solution, proper choice of membrane material/ properties, appropriate setting of the operational parameters and the use of turbulence promoters. Although these approaches reduce fouling to some extent, almost all fi ltration applications require a chemical cleaning step after a given length of time. Membrane separation processes can be operated in cross-flow (i.e., feed flow along the membrane surface) or dead-end (i.e., feed flow perpendicular to the membrane surface) mode. |
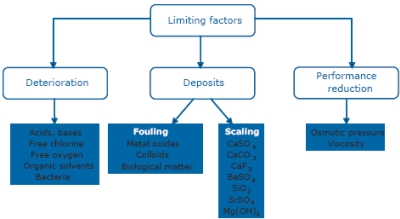 |
Cross-flow mode induces turbulence at the membrane surface to inhibit the build up of the fouling layer on the membrane surface. In dead-end filtration, retained particles accumulate to form a cake layer and fouling tendencies are therefore high. Microfiltration and Ultrafiltration technology Microfiltration (MF) closely resembles conventional coarse filtration and concerns the separation of particles between 0.1 and 10 μm, such as suspended solids (colloids), bacteria and large proteins. It is applied for clarification and sterilization purposes, for cell harvesting, separation of oil-water emulsions, etc. MF employs membranes with a porous structure corresponding to low operating pressures in the 0.1 to 2-bar range. Ultrafiltration (UF) retains relatively large molecules such as proteins, polymers and colloidal substances. Small molecules such as salts pass through the membrane intact. UF is ideally suited for fractionation, concentration and purification purposes. Operating pressures are typically in the range of 1 to 5 bar for cross-flow application. With a semi-dead end operation mode, the pressures are much lower, around 0.2-0.3 bar (see section on water treatment). MF and UF membranes can be made of hydrophobic polymers (polyfluoroethylene, PTFE or Teflon; polyvinylidene difluoride, PVDF or polypropylene, PP), hydrophilic polymers (cellulose esters; polysulfone, PS; polyethersulfone, PES) or inorganic ceramics. The selection of the membrane material often occurs on the basis of the potential interaction of the membrane material with the feed stream. In any case, periodical chemical cleaning of the membranes will be needed. In this respect, membrane stability over a wide pH range and stability in the presence of active chlorine are important requirements. In addition, particularly in biotechnological applications, stability in the presence of steam sterilisation is also important. For the latter applications, polyethersulfone (PES) is often preferred for its higher temperature resistance. New developments The main developments in MF/UF aim at obtaining higher selectivity, the removal of finer particle sizes, lower energy consumption and/or operation for longer periods of time before cleaning or disposal. The continuous reduction in membrane cost per m² allows more membrane surface area to be used, making a corresponding shift to lower permeate fluxes possible, thus increasing membrane life and long-term membrane performance. |
Other developments are related to advances in polymer chemistry, to membranes with improved chemical and fouling resistance and a longer lifetime, and to the introduction of ceramic membranes in the water-treatment market. Increasing attention is being paid to immersed membranes for large plants due to their smaller footprint and hence lower capital costs (see also the section on MBR). In the longer term, major developments are expected in the area of membrane functionalisation, e.g. by modification with ligands, by a combination with enzymes and/or nanoparticles. |
 |
Reverse Osmosis / Nanofi ltration Reverse osmosis (RO) is typically used to concentrate, purify and recover valuable components, either alone or in combination with other separation processes such as MF and UF or evaporation. For RO, water molecules are forced through a dense membrane by applying a pressure higher than the osmotic pressure of the feed stream, allow the removal of nearly all ions and organic compounds. Operating pressures can range from 10 bars up to 100 bars. A typical RO application is seawater desalination. |
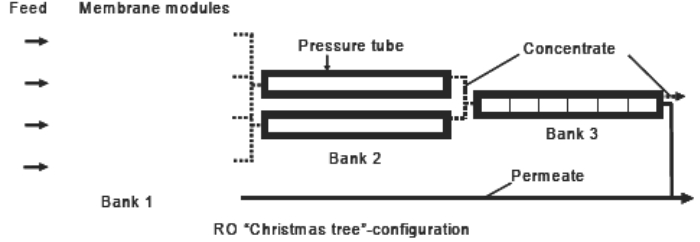 |
RO membranes typically are integrally skinned, asymmetric membranes or thin-film composite membranes with a layered structure. Nearly all RO installations include horizontally located, spiral-wound membrane modules. The modules are standardized and fit in pressure tubes. RO installation design involves the use of multiple membrane modules in series, placed in parallel in so-called banks. Nanofiltration (NF) typically retains multivalent ions, such as phosphate or sulphate, and molecules with molecular weights above 200 Dalton. Monovalent ions are only partially retained (typically 50 %). Consequently, NF membranes are useful in retaining Chemical Oxygen Demand (COD, a measure of the organic load of wastewater), for softening, and for partial demineralization or removal of salts, particularly when monovalent ions such as chlorides do not have to be removed. Operating pressures are between 5 and 20 bar. Most of the NF membranes are composite membranes, and suitable module configurations are capillary and spiral wound. Operation is in cross-flow mode. New developments The two major (emerging) trends for RO for the past 15 years are improved performance and a signifi cant reduction in price. Ongoing developments are mainly linked to ultra-low pressure composite membranes and optimized module hydrodynamics. From an energy point of view, special attention is paid to combining with thermal technologies to obtain hybrid desalination systems. A very recent line of research focuses on salinity gradient power (SGP) production. This technology is able to convert the osmotic energy of salt solutions into mechanical or electrical energy. Two main SGP techniques are currently available: reverse electrodialysis (RED) and pressure retarded osmosis (PRO). For NF, recent developments have greatly extended the capabilities of the membranes to withstand aggressive environments. For water-based applications, these can be very high or very low pH environments. For most organic solvents, traditional membranes lose their stability or underperform with respect to flux or rejection. Consequently, organic solvent resistant membranes − both ceramic and polymeric − have been developed which find application in solvent exchange, solvent recovery, catalyst recovery, product purification, etc. Further progress was also made on improved performance with regard to both permeability and selectivity. Because multivalent ions are retained and monovalent ions are not, NF membrane retention can be tailored to specific applications. The use of ion-selective NF membranes as a pretreatment for RO feed water for example can be an option in reducing the scaling potential. This would allow higher system recoveries and lower chemical demand in the RO stage. |