 |
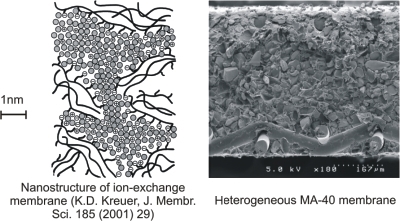 |
Ion exchange membranes (IEM) are nanoporous materials. The structure of the membrane can be represented as a hydrophobic polymer matrix containing within itself a network of hydrophilic channels, the walls of which bear an electrical charge. The distance between the walls of the channels is a few nanometers, the ions with the charge of the same sign as the walls (co-ions) are pushed out by Coulomb forces. The wall charge causes filling of the channels with water (or other solvent) and charge carriers: ions of opposite sign of charge (counterions). This structure determines permselectivity of the membrane to counterions: cations in cation-exchange or anions in anion-exchange membranes. Among commercial monopolar ion exchange membranes, they distinguish homogeneous membrane with the structural heterogeneity at the nanometric level (e.g. Nafion), and heterogeneous membranes (e.g. MA-40), in which the particles of ion exchange resin (having a structure similar to the homogeneous membranes) with a size of 10-50 microns are distributed in an inert binder (e.g. polyethylene). Bipolar membranes are of particular interest. These membranes are composed of a cation-exchange and an anion-exchange layers. In an imposed electric field, cations and anions move from the bipolar interface to the external solution; that results in depletion of the interface in relation to the salt ions. Then the charge transfer is ensured by new carriers, the ions H + and OH-, which are generated by water splitting reaction. The reaction proceeds in a thin boundary layer of thickness 1-2 nm, where it is accelerated by the catalytic participation of functional groups or specially introduced catalysts, as well as by a strong electric field inside the electrical double layer. |
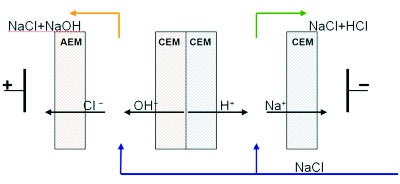 |
The generation of ions H + and OH- in ED cell with bipolar membrane allows one to obtain solutions with controlled pH value up to concentrated (2-3 M) solutions of acids and alkalies. |
Ion-exchange membranes (IEM)
and electro-membrane technologies |
IEMs are used in analytical chemistry (sensors, electro -chromatographic separation); for the selective transfer of protons (fuel cells) and in microfluidics (micropumps, electrophoresis), but the most important application is electrodialysis. |
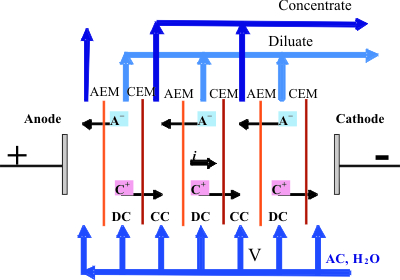
Electrodialysis is a separation process consisting in simultaneous removal of cations (C+) and anions (A-) from an aqueous (or organic) solution. C+ passes through cation-exchange membrane (CEM) and (A-) through an anion-exchange one (AEM) under the action of an electric field. These membranes form a desalination (DC) and concentration (CC) channels. The final product may be a solution with modified ionic composition, desalted solution and (or) concentrate. Hydraulically isolated CC are used for extremely concentrated (close to saturation) solutions. Recently, hybrid technologies including ion-exchange, baromembrane (microfiltration, MF, reverse osmosis, RO) and electro-membrane processes seem to be the most efficient. |
 |
Towards ZLD (zero liquid discharge) technologies The ions of hardness are removed by sodium cation exchange. The demineralization up to a concentration of 10-50 mg / L is realized using a RO module. The remaining ionic impurities are extracted in an EDI module with simultaneous adjustment of pH: ion-exchange filling and / or profiled membranes are applied. The retentate after RO is additionally concentrating by an ED-concentrator. Since the feed water is pre-soften, the risk of precipitation is absent. A part of the concentrate is used for the regeneration of ion-exchange columns. The salt concentration can be increased up to 150-200 g / L, thus reducing the volume of liquid effluent <1% of the raw water. |
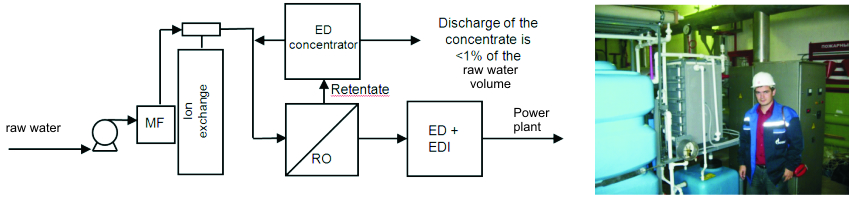 |
This process flowsheet shows the hybrid membrane complex for the production of feed water production at a heat power plant. The set up is elaborated by "IE Membrane Technology" (Russia) and JSC "Membrane Technologies" (Kazakhstan). |
Electrodialysis (ED) is used for desalination, demineralisation (e.g. milk and whey) and the removal of metals. In these applications, ED often competes with ion exchange (IEX), but the continuous process operation of ED makes it more economical. An ED installation consists of a stack of alternating anion-conductive (AC) and cation-conductive membrane (CM). When an electrical field is applied the anions will move to the anode through the AM while the cations move through the CM towards the cathode. This generates a concentrated stream and a diluted stream. Opposite to RO, ED involves desalination that transports only the solute (ions) through the membrane. ED thus needs less material transport. The amount of ions transported is directly proportional to the electrical current or current density. With respect to fouling, a very important method to counteract the deterioration of membrane performance by foulants is ED reversal (EDR). During ED reversal, the anode switches to cathode and vice versa, while the diluate and concentrate streams are also interchanged. Such reversal is typically performed every 30 to 60 min. The production “loss” involved is only a small percentage. ED reversal should not be confused with reverse ED (RED), which is a new technique to generate power from a salinity gradient. |